|
Dr. F. Parodi - Industrial R&D Expert technical web papers: # 2 |
|
Dr. F. Parodi - Industrial R&D Expert technical web papers: # 2 |
|
Fast-Curing and High-Performance Isocyanate–Epoxy FPR Resin Systems |
| for Structural Composites |
| and Heavy-Duty Electrical/Electromechanical Applications |
|
Fabrizio Parodi |
Isocyanate–Epoxy FPR Resin Systems are proprietary products of Dr. F. Parodi
__________________________________________________________________________________________
|
CONTENTS 1. High-Performance Thermosetting Resins 2. Poly(isocyanurate)s and Poly(2-oxazolidone)s 3. Fast-Curing and High-Performance Isocyanate-Epoxy FPR Resin Systems 3.2 Processability Characteristics 3.1a Pot-life and polymerization rate 3.1b Microwave processability 3.1c Rheological properties 3.3 Properties of Cured Resins 3.3a Distortion temperature 3.3b Thermo-oxidative resistance 3.3c Fire resistance 3.3d Water uptake and chemical resistance 3.3e Mechanical and thermo-mechanical properties of neat, cured resins 3.3f Manufacturing and properties of structural composite materials 3.3g Electrical properties 4. Main Grades of Isocyanate-Epoxy FPR Resin Systems Developed 4.2 Specialty and Proprietary FPC Curing Catalysts
|
___________________________________________________________________________________________
| ______________________________________________________________________________ | ||||||
| Table 1a - Conventional Thermosetting Resins: Tg and price index values | ||||||
|
______________________________________________________________________________ |
||||||
|
|
|
ortophthalic | Tg = 90 ÷ 100°C | price index = 1.0 | ||
|
unsaturated polyesters |
isophthalic | Tg = 115 ÷ 125°C | price index = 1.1 ÷ 1.2 | |||
|
|
bisphenolic | Tg = 110 ÷ 130°C | price index = 1.2 ÷ 1.4 | |||
|
|
||||||
|
vinyl-esters |
|
standard | Tg = 120 ÷ 130°C | price index = 2.7 ÷ 3.2 | ||
| multifunctional | Tg = 160 ÷ 185°C | price index = 3.5 ÷ 4 | ||||
|
|
||||||
|
epoxy resins + standard hardeners |
|
standard |
|
Tg = 120 ÷ 165°C | price index = 2.8 ÷ 3.5 | |
| epoxy-novolacs | price index = 4.8 ÷ 5.5 | |||||
|
|
||||||
|
phenolics |
||||||
|
amino-resins (urea-formaldehyde, melamine-formaldehyde, etc.) |
||||||
|
______________________________________________________________________________ |
||||||
| ________________________________________________________________________________ | |||||
| Table 1b - High-Performance Thermosetting Resins: Tg and price index values | |||||
| ________________________________________________________________________________ | |||||
|
conventional epoxy resins & epoxy-novolacs + specialty hardeners |
|
Tg = 180 ÷ 280 °C | price index = 4.5 ÷ 6.5 | ||
|
|
|||||
|
specialty multifunctional epoxy resins + specialty hardeners |
|
Tg = 260 ÷ 340 °C |
price index = 8 ÷ 15 |
||
|
|
|||||
|
condensation polyimide resins |
Tg > 450 °C |
|
|||
|
PMR polyimide resins |
Tg = 400 ÷ 450 °C |
price index = > 60 |
|||
|
bismaleimide resins (std.) |
Tg = 350 ÷ 400 °C | ||||
|
|
|||||
|
polystyryl-pyridine resins |
|||||
|
acetylen- (or ethynyl-) functional |
|||||
|
benzocyclobutene resins |
price index = 20 ÷ 50 |
||||
|
cyanato-functional resins |
|||||
|
N-cyanoureido-functional resins |
|||||
|
|
|||||
|
ISOCYANATE-EPOXY resins FPR S (standard grades) |
Tg = 270 ÷ 300 °C |
price index = 3.6 ÷ 4.5 |
|||
|
ISOCYANATE-EPOXY resins FPR H (specialty grades) |
Tg = 300 ÷ 320 °C |
price index = 4.3 ÷ 5.0 |
|||
|
________________________________________________________________________________ |
|||||
|
Because of the complex chemistry and/or the expensive organic chemicals involved, such high-performance resins are affected by price levels, in practice from 4-20 times higher then those of the best conventional resins. This still confines their industrial uses to the narrow fields of composite materials for missile, military, aeronautical and aerospace constructions, as well as to specialized electrical & electronic devices and components, whose very critical service conditions had dictated the development of most high-performance resins during the 1970s and 1980s. Besides their heavy economics, intrinsic and often remarkable processing issues are proper to these resins: i) many of them are solids to be hot-melted and kept warm during all the processing stages; ii) many of them are extremely viscous liquids, whose manipulation is feasible only under adequate heating to moderate their viscosity, or even preferably as solutions in organic solvents (to be stripped thoroughly away after, e.g., the fiber impregnation operations as in the manufacturing of pre-pregs for structural laminates or printed circuit boards); iii) the existing high-performance resins are inherently characterized by slow curing kinetics, requiring prolonged processing times at high temperatures (hardening temperatures typically above 150°C, followed by long post-curing treatments at 200-300°C, and even higher temperatures). |
|
|
|
|
|
Numerous polymeric products containing (chemically and thermally-stable) heterocyclic chemical structures (exemplified in Scheme 2) are attainable from organic isocyanates through a plurality of cycloaddition or cyclocondensation reactions [5]. Among such polymeric products, poly(isocyanurate)s have a renowned industrial importance: typically glassy, densely cross-linked and brittle polymeric materials containing a plurality of isocyanurate structures (A), largely employed as rigid cellular materials for thermal and/or acoustic insulation. Such products are attainable by the direct, and optionally very fast, cyclotrimerization, promoted by a variety of catalysts, of liquid polyisocyanates and/or isocyanato-functional oligomers [5]: Equation (1) of Scheme 3].
|
|
|
|
|
|
In parallel to poly(isocyanurate)s, R&D efforts were devoted years ago to poly(2-oxazolidone)s, thermoplastic polymers with a chemical structure comprising the disubstituted heterocyclic (penta-atomic) oxazolidine-2-one (or simply 2-oxazolidone) structures (B.1) and/or (B.2). These products can be conveniently synthesized through the cycloaddition reaction, activated by suitable catalysts, between isocyanates and epoxides shown as Equation (2) in Scheme 3. |
|
|
|
|
|
|
| (a) complete TTT diagram for a generic thermosetting resin |
(b) complete CHT diagram for a generic termosetting resin |
|
|
Figure 1 - Transformation diagrams for thermosetting resins: a) for isothermal curing treatments [Time–Temperature–Transformation diagrams (TTT diagrams)]; b) for curing treatments under continuous heating, at constant heating rate [Continuous Heating Transformation diagrams (CHT diagrams)]. |
||
|
|
|
Figure 2 - TTT diagram of the std. Isocyanate–Epoxy FPR S-1 resin (medium-slow catalysis). |
 |
|
Figure 3 - Transformation diagram under continuous heating, at constant heating rate [Continuous Heating Transformation diagram (CHT diagram)] of the specialty Isocyanate–Epoxy FPR H-0 resin (slow catalysis). |
|
|
|
|
|
Figure 4 - Process stages of the overall dynamic curing of the specialty Isocyanate–Epoxy FPR H-1 resin (with slow catalysis), as evidenced through the evolution of its dynamic-mechanical properties (shear moduli G' and G") under a linear heating ramp at 2°C/min up to 360°C. |
|
3.2b Microwave Processability Thanks to the peculiar physico-chemical properties and chemical mechanisms of action of their specialty catalysts, these isocyanate-epoxy resins are exceptionally-well suited to be cured and/or post-cured by microwave heating. By means of such processing method, the curing and/or post-curing times can typically be minimized to 1/4 ÷ 1/10 of those required under conventional thermal conditions at the same temperature [10-12]. For instance, the 2 hour-long post-curing cycle at 180-220°C of (S-RIM-molded) glass fiber-reinforced FPR S-1 plates can be accomplished in just 15 minutes under microwave heating at an average temperature of 225°C of the resin plates. Novel proprietary catalysts, specifically developed for the microwave processing of FPR resins ( FPC W1 e FPC W2 ), allow for the preparation of FPR resin compositions endowed with the following, extremely interesting combination of features: a prolonged pot-life at room temperature (up to 4-6 hours), coupled with particularly short vitrification times under microwave irradiation minimized to 1/8 - 1/10 of those under conventional thermal treatments, at the same resin temperature. |
|
3.2c Rheological Properties
|
|||||||||||||||||||
|
3.3 Properties of Cured Resins
3.3a Heat Distortion Temperature
Glass Transition Temperature ( Tg ~ HDT ). Depending on the resin formulation: Tg of standard ISOCYANATE-EPOXY FPR S resins = 250 ÷ 300°C (typically: 270 ÷ 300 °C); Tg of specialty ISOCYANATE-EPOXY FPR H resins = 300 ÷ 320 °C. Plasticized formulations possess a glass transition temperature lowered to180 ÷ 240°C; for partially plasticized grades, the Tg spans the 230 ÷ 270 °C range. As an example of this, the dynamic-mechanical spectra of Figure 6 display a Tg ~ 300°C for the standard FPR S-1 resin (in excellent agreement with the value of ~ 290°C resulting from DSC analysis), and a Tg = 265-275 °C for the partially plasticized, low-viscosity FPR S-1 LV resin. |
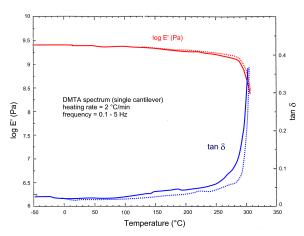 |
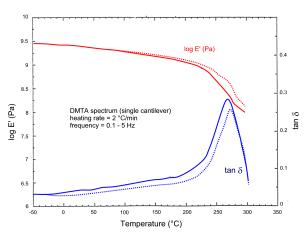 |
|
|
(a) std. FPR S-1 resin (HDT > 250°C) |
(b) partially plasticized FPR S-1 LV resin (HDT = 230-240 °C) |
|
|
Figure 6 - Dynamic-thermal-mechanical (DMTA) spectra, under 2°C/min heating scans, for two neat, fully cured, Isocyanate–Epoxy FPR resins. |
||
|
3.3b Thermo-Oxidative Resistance Fully cured FPR resins possess an excellent thermal stability up to 280°C, being currently able to sustain continuous use temperatures > 150°C (typically, of 160 ÷ 180 °C, and up to 200°C), and peak temperatures up to 350-360 °C. Their high-temperature aging, in both inert atmosphere and air, implies a smooth and slow weight loss, without any bulk and surface micro-structural damages (with the resin surface remaining in fact smooth and brilliant). For example, the weight loss of neat resin specimens is of 5 - 6 % after 200 hours at 250°C in air; and that of glass fiber-reinforced or mineral powder-filled resins (i.e., quartz-, calcined clay-, silica-filled, etc.) [60 wt. % of fibers or mineral powder] spans the 2.5 - 2.8 % interval after 2000 hours of continuous exposure at 200°C in air. To give a better picture of this, Table 4 compares the thermo-oxidative resistance of FPR H resins with that of qualified, current epoxy resin thermosets. The data of Table 4 show a remarkable superiority of FPR H resins, even with respect to anhydride-hardened epoxy resins, whose thermo-oxidative resistance is currently acclaimed as optimal among thermoset polymeric materials, and weaker only than the much more expensive imide, cyanato- and ethynyl-functional resins. |
|
Table 4 - Long-Term Thermo-oxidative Resistance of ISOCYANATE-EPOXY FPR Resins as Compared to Qualified, Current Epoxy Resin Thermosets: % weight loss under continuous exposure to hot air (specimens of neat, fully crosslinked resins). |
||||
| %
weight
loss
after |
FPR H resins | DGEBA, DGEBF & epoxy-novolacs | epoxy-novolacs
+ DDS |
|
| + std. anhydrides | + std. amines | |||
| 200 h at 210°C | 0.3 ÷ 1.3 % | 2.5 ÷ 5.8 % | ||
| 100 h at 260°C | 4.8 ÷ 6.1 & | > 6 % * | ||
| 200
h at 250°C
200 h at 260°C |
5
÷ 6 %
|
9.0 ÷ 10.5 % |
> 10 % * |
> 10 % * |
| Tg of the various materials | 300 ÷ 320 °C | 140 ÷ 220 °C | 130 ÷ 220 °C | 210 ÷ 255 °C |
|
* associated with significant to strong oxidative degradations |
||||
|
3.3c Fire Resistance Fully-cured, neat FPR resins are inherently flame retardant (with respect to neat unsaturated polyester, vinyl-ester and conventional epoxy resins), and display a fire behavior not so much different from that of phenolic and imide resins. Classification according to the UL 94 test method (Underwriters Laboratories), for 3.2 mm-thick specimens:
|
|
3.3d Water Uptake and Chemical Resistance
|
||||||||||||||||||||||
|
Table 6 - Long-Term Chemical Resistance of ISOCYANATE-EPOXY FPR Resins as Compared to Qualified, Current Epoxy Resin Thermosets. |
||||
| resistance | FPR H resins | DGEBA, DGEBF & epoxy-novolacs | epoxy-novolacs
+ DDS |
|
| + std. anhydrides | + std. amines | |||
|
hydrolytic |
"unlimited" | very critical | good | good |
|
to mineral oils up to 200°C |
excellent | good | very good | very good |
|
to common organic solvents |
very good | good | very good | very good |
|
to strong bases (diluted) to strong bases (concentrated) |
excellent
very good |
poor
very poor |
very
good
good |
excellent
very good |
|
to strong acids (diluted) to strong acids (concentrated) |
excellent
very good |
good
fair |
poor
very poor |
critical
poor |
|
to nitric acid & nitric mixtures |
fair | poor | very poor | poor |
|
Tg of the various materials |
300 ÷ 320 °C | 140 ÷ 220 °C | 130 ÷ 220 °C | 210 ÷ 255 °C |
|
3.3e Mechanical and Thermo-Mechanical Properties of Neat, Fully-Cured Resins
A comparison of these values of flexural properties with the variability intervals for different types of high-quality epoxy thermosets (given in Table 7) shows a comparable rigidity, and a realistically ~ 20%-lower strength of FPR resins [for dry-conditioned specimens at 23°C (at a relative humidity of 50-55 %)]. However, because of the higher-to-much higher water uptake attitudes of epoxy thermosets with rispect to FPR resins (as said in paragraph 3.3d and shown in Table 5), comparative characterizations performed on samples conditioned for long times at 23°C at a relative humidity of 95-100 % evidenced a general equivalence of flexural and tensile strength between epoxy thermosets and FPR resins, and significantly higher values of elastic moduli of cured FPR resins with respect to the same reference epoxy materials.
|
|
Table 7 - Mechanical Properties of ISOCYANATE-EPOXY FPR Resins as Compared to Qualified, Current Epoxy Resin Thermosets: flexural characteristics at 23°C according to ASTM D790 (materials dry-conditioned at 23°C) |
||||
| property | FPR S & H resins | DGEBA, DGEBF & epoxy-novolacs | epoxy-novolacs
+ DDS |
|
| + std. anhydrides | + std. amines | |||
| flexural
strength (MPa) |
90 ÷ 110 | 125 ÷ 145 | 90 ÷ 130 | 120 ÷ 140 |
| flexural
modulus
(GPa) |
3 ÷ 4 | 3.2 ÷ 3.5 | 2.6 ÷ 3.4 | 3.2 ÷ 3.4 |
| Tg of the various materials | 270 ÷ 320 °C | 140 ÷ 220 °C | 130 ÷ 220 °C | 210 ÷ 255 °C |
|
3.3f Manufacturing and Properties of Structural Composite Materials
|
|
resin feeding |
► |
|
|
|
Figure 7 - Optical macro-photograph depicting the evolution of the vacuum infusion of the glass-fiber filling (4 plies of woven-roving, 345 g/sqm each) of a Bag Molding envelope by the FPR S-1 resin (with slow catalysis, at room temperature). Back + front lighting. |
|||
|
resin feeding |
► |
|
|
||
|
(a) front lighting |
(b) front lighting |
||||
|
|
|
|
|||
|
(c) back visible lighting + front UV lighting (Wood lamp) |
(d) back lighting |
(e) back lighting |
|||
|
Figure 8 - Optical stereo-micrographs showing the evolution of the vacuum infusion of the glass-fiber filling (4 plies of woven-roving, 345 g/sqm each ) of a Bag Molding envelope by the FPR S-1 resin (with slow catalysis) at room temperature: virgin and fully impregnated fibers, and advancing front of the liquid resin. |
|||||
quasi-isotropic 8-ply glass fiber-reinforced laminates (0, 90, ±45°, symmetrical); properties at 23°C
orthotropic 8-ply glass fiber-reinforced laminates (0, 90°); properties at 23°C
The excellent mechanical properties of fiber-reinforced FPR thermosets are linked primarily to the high adhesion of such resins to reinforcing fibers, as evidenced by the SEM micrographs of Figure 9, showing the fracture morphology of glass fiber-reinforced FPR resins. These micrographs exemplify, with both standard and partially plasticized FPR matrices, the post-impact permanence of significant portions of the glassy polymeric matrix firmly linked to the fiber surfaces, despite the micro-morphology proper to a brittle fracturing of the composites. Such strong FPR resin-fiber adhesion is further demonstrated by the values of the interlaminar shear strength (short-beam shear strength, according to ASTM D2344) of glass and carbon fiber-reinforced FPR resin orthotropic laminates (at 23°C):
|
|
|
|
|
|
|
||
|
(a) polymer matrix: standard FPR S-1 resin |
||
|
|
||
|
|
|
|
|
|
||
|
(b) polymer matrix: partially plasticized FPR S-1 resin |
||
|
Figure 9 - SEM micrograph pictures of the fracture morphology of glass fiber-reinforced Isocyanate–Epoxy FPR resins after a destructive ball-drop impact at room temperature. RTM composites; fiber reinforcement: woven roving. |
||
|
3.3g Electrical Properties The FPR resins are per se characterized by a spectrum of electrical properties (i.e., dielectric strength, dielectric constant and loss factor, surface and volume resistivity, as well as thermal endurance) similar to that of the best epoxy materials qualified on the market for heavy-duty, low and medium-voltage electrical applications (high-power electrical transformers, big capacitors and insulators, etc.). Cast thermosets from quartz powder-filled (60 wt. %) FPR H-0 and H1 resins exhibit the following, typical properties:
|
|
4. Main Grades of Isocyanate-Epoxy FPR Resin Systems Developed
4.2 Specialty and Proprietary FPC Curing Catalysts All the FPC Catalysts are moisture-insensitive, non-toxic, non-noxious, non-corrosive, and nonflammable compounds, with a minimum shelf life of one year at room temperature when properly stored in closed containers, and kept protected from prolonged exposure to sunlight or artificial actinic light (at best, in metal drums or cans, or also in brown glass vessels). "Pure catalyst" grades are available together with complete FP Resin Systems only. "Catalyst concentrate" grades can be supplied separately from isocyanate and epoxy resin components of FP Resin Systems, liquid isocyanates and epoxy resins being directly purchased on the local market by the user according to our specifications for the various FP Resin Systems.
|
|
|
Isocyanate–Epoxy FPR Resin Systems are proprietary products of Dr. F. Parodi
_______________________________________________________________________________
|
© Dr. Fabrizio Parodi - 2000-2015 |