|
1. White & Colored, Traditional Vulcanized Elastomers vs. Thermoplastic Rubbers The uses of white and colored rubber compounds [1] are currently spread over a myriad of applications:
Similarly to traditional black rubbers [1,2] but at a greater extent, white & colored ones are fighting from many years with thermoplastic elastomers or elastoplastic materials [3], primarily with TPUs (thermoplastic polyurethanes), and TPV materials (that is, fine dispersions of dynamically vulcanized elastomers in plastic matrices, such as vulcanized EPDM or butyl rubber - polypropylene blends, and vulcanized nitrile rubber - nylon blends, such as Santoprene ®, Dytron XL ®, Trefsin ®, and Geolast ® thermoplastic rubbers, proprietary products of Advanced Elastomer Systems, L.P.). As known, TPVs and TPUs present a couple of remarkable advantages over conventional rubbers: a) a practically unlimited shelf-life; b) the fact of being easily and fully recyclable by simple grinding, melt-extrusion and pelletization. On the contrary, mechanical and thermal properties of vulcanized rubbers in general are still quite advantageous over those of TPVs and TPUs (fairly lower tensile and compression permanent set values, superior heat resistance and dimensional stability, much better elastic recovery, lower brittle temperature, the possibility of considerably higher fractions of mineral fillers, the widest choice of foaming agents and foaming procedures applicable for the generation of sponged materials, a generally better resistance to organic solvents and aggressive chemicals, etc.) [3]. In this competition, the major drawback of conventional rubbers, with respect to thermoplastic elastomers, is the need of the time-consuming and/or labor-intensive, and energy-consuming, heat treatments required to accomplish their thermally-activated chemical crosslinking [4], also involving the investment and maintenance costs of related vulcanization tunnel or closed-chamber furnaces and ancillary equipment as additional charges [5]. For TPVs and TPUs, the plant investment and manufacturing cost savings (associated with production rate gains) linked to the absence of thermal treatments following their molding or extrusion stage may often be so remarkable to encompass the immediate advantage of the lower prices of comparable, traditional rubber compounds. Therefore, the only way to offer finished parts with the superior end-properties of vulcanized rubbers at prices comparable to those of TPVs and TPUs would be to adopt or devise strong processability and productivity enhancements of conventional rubber vulcanizates. In this respect, microwave (UHF) power vulcanization technology is recognized to have an unparalleled potential as the most efficient and cheapest processing option to get to this goal [6-8]. Microwave processing offers indeed a widely acclaimed and unique range of advantages over all the other heat-processing technologies applicable in the rubber industry. Such advantages essentially come from the peculiar energy transfer mechanisms of this heating technique, implying a volumetric, deeply penetrant and fast heating of dielectric materials irrespective of their low thermal conductivity, resulting in particularly high heating rates; heating rates which, for thick objects, may be 10-50 times higher than those attainable by any other heating method known. This makes microwave processing an absolutely unrivaled method for the manufacturing of thick and heavy rubber articles, where all the other vulcanization techniques based on external heat transfers (conductive heating) are very slow and volumetrically inhomogeneous [5-8].
2. Advantages of Microwave Vulcanization over the Other Existing Technologies The principal advantages of microwave technology over the other rubber vulcanization methods are summarized in Table 1 below [5-8]. Table 1 - Microwave (UHF) Vulcanization vs. the Other Rubber Processing Methods
Microwave processing (namely, the UHF tunnel vulcanization of continuous extruded profiles, beams, hollow and contoured bars) is a well established reality in the manufacturing of conventional, carbon black-filled rubber articles [6-8], with productivity levels and manufacturing costs contributing to keep these vulcanized elastomers significantly competitive versus their thermoplastic analogues. By contrast, for the reasons and in the terms outlined below, most vulcanizable white & colored rubber compounds cannot, and actually do not, exploit at present this valuable technological opportunity, thus being more and more pressed on the market by their thermoplastic competitors. In this context, a novel, entire class of proprietary UHF heating susceptors, specifically designed and under development at fpchem.com as colorless additives for white & colored rubber compounds, offers a technological breakthrough to make most, normally microwave-transparent or just feebly microwave-absorbing materials of this category really and conveniently processable by microwave energy, in this way contributing to the possibility of finished articles with manufacturing costs comparable to those of the well-established black-rubber vulcanizates, and overall, raw materials + manufacturing costs competitive with those of the best thermoplastic analogues.
3. Present Limitations to an Efficient and Extensive Use of Microwave Vulcanization for White & Colored Rubber Compounds In contrast to black rubber compounds, where the electrically conductive carbon black filler plays a key role as a powerful and cheap microwave (and radio frequency) absorption susceptor, most white & colored ones display the negligible-to-poor microwave heatability proper to their typical ingredients: the most important (nonpolar) neat rubbers (including EPDM, natural rubber and synthetic polyisoprenes, butyl rubber, and polybutadienes), paraffinic or aromatic oils, usual white mineral fillers (native and calcined kaolins, calcium carbonates, talcs, silicas, etc.) and pigments (such as titanium dioxide) . With the comparatively minor exception of those based on polar rubbers inherently possessing an acceptable-to-fair microwave heatability (polychloroprene, polyepichlorohydrin, nitrile rubbers, chloro- and bromo-butyl rubbers), the generality of white & colored rubber compounds are unsuitable at all, or at best scarcely suitable, to be processed in UHF tunnels and ovens [7-10]. The organic additives used to date (such as polyoxyethylene-glycols, related ester, ether and amide derivatives, metal soaps, salts of fatty acids with amines or hydroxyalkylamines, esters and amides of fatty acids, dialkyl phthalates, phosphate esters, modest amounts of polar rubbers, etc.) display a really scarce effectiveness, and allow for a microwave heatability and processability (i.e., heating and chemical crosslinking rates) of most, ordinary white & colored rubber compounds being at best 20-25% of those of conventional carbon black-filled ones. Several dispersed mineral fillers may induce improvements by various mechanisms of high-frequency electromagnetic energy dissipation, e.g. through Maxwell-Wagner interfacial polarization (particulaly as in the case of untreated and silane-treated silicas), or via electronic conduction (as with certain grades of zinc oxide); however, because of the predominant role of such phenomena in the radio frequency region, these additives are again largely ineffective at the UHF frequencies of practical interest in this field [7]. Moreover, many additives among those mentioned above are either detrimental to the performance of finished rubber articles, as in the cases of polyoxyethylene-glycols and many water-soluble polyoxyethylene derivatives (easily extractable from rubber vulcanizates by aqueous media), the same polyoxyethylene-glycols, all polyoxyethylene derivatives, and salts or amides from hydroxyalkylamines (all of them remarkably hygroscopic, and thus causing undesirable water or moisture uptake problems by finished rubber vulcanizates), or often unwanted components because of related safety and/or ecological issues (i.e. dialkyl phthalates, many phosphate esters, and all chlorinated and brominated polar rubbers). For such reasons, only a minute fraction of the white & colored rubber compounds manufactured worldwide per year is currently being processed by means of UHF heating treatments. In all cases, rubber manufacturers insisting on the use of UHF vulcanization technology for white & colored rubber articles are currently struggling with the heavy productivity limitations and remarkable heating energy losses proper to this kind of rubber compounds. Moreover, the very poor microwave absorption attitudes of present white & colored rubber compounds imply abnormally high values of the ratio between the reflected and absorbed electromagnetic power in UHF ovens, with an effective usage of the total power input not so rarely of the order of 10%, or even lower. The largest portion of the reflected power is being conveyed to the dummy loads of microwave systems, and entirely dissipated (and lost) via their water cooling circuits; part of it typically goes to burn or damage the metal seals and electromagnetic guards of microwave tunnels, causes harmful electrical glow discharges, shrinks the life of the electronics of microwave generators, etc. Often, in fact, rubber vulcanizates made of compounds of this type exit from UHF tunnels being used in a totally inappropriate way, as a sort of expensive, not so much efficient, and even harmful, hot-air furnaces! Similar problems are also being displayed by many conventional rubber compounds based on the (cheaper) carbon blacks characterized by a modest electrical conductivity.
4. The Novel fpchem.com's Microwave Heating Susceptors for White & Colored Rubbers An R&D program was launched by Dr. Parodi in 2003 on a novel category of specialized, colorless microwave absorption enhancers for white rubber compounds, involving molecular design, organic chemistry research, physicochemical characterizations and microwave heating testing at fpchem.com, and laboratory and pilot preparations of rubber compounds, + related extrusion & UHF processing trials, at external rubber facilities. Two types of such novel additives have been devised, at present at different research and/or development stages as explained in the following: a) chemically inert microwave heating susceptors [XS and XN additives]; b) rubber-coreactive microwave heating susceptors [XSR and XNR additives]. Both chemically inert and rubber-coreactive microwave heating susceptors are suitable for all the applicable rubber vulcanization methods, i.e. sulfur, peroxide or phenolic resin-crosslinking rubber formulates.
4.1 General Physical & Chemical Properties of the Neat Additives Synthetic, super-dipolar organic chemicals, with the following properties:
4.2 General handling, storage and safety characteristics
The novel microwave absorbers are easily absorbed by mineral fillers currently in use in the rubber industry, and exceptionally well by calcined kaolins and silicas (sub-micronized talcs and micas may also be suitable). Mineral powders act as excellent carriers of the additives, make their own homogeneous dispersion within rubber compounds much easier and faster.
5. Technical Performance, Costs & Benefits of the Novel Microwave Heating Susceptors The novel additives can be added directly to rubber compounds during their Banbury mixing, preferably just prior to the addition of vulcanizing agents and accelerators. They may also be added to rubber compounds in open mixers. Since the boiling points of the novel additives are in excess of 300°C, and their vaporization enthalpy is very high as well, their vapor pressure remains negligible at the ordinary rubber compounding and extrusion temperatures of 80-120°C, and particularly low at the usual vulcanization temperatures of 180-220°C. Chemically unreactive XS and XN additives affect only minimally (and usually negligibly) the thermal vulcanization rates, and at a little extent the rheological properties, of the white & colored rubber compounds to which they have been added. The thermal vulcanization curves of Figure 2 (at 170°C) exemplify this for a standard, sulfur-vulcanizable, white EPDM rubber compound without (Figure 2a), and containing 5% by weight (~20 phr) of the XS_04 additive (Figure 2b), respectively. The somewhat lowered melt-viscosity of rubber compounds and the stiffness of vulcanizates (ascribed to simple dilution effects of XS and XN additives, similar to those exerted by oil extenders) may by adjusted through modest compositional corrections.
|
|||||||||||||||||||||||||||||

|
Figure 2a - Standard white EPDM rubber compound.
torque max = 32.95 lb·in torque min = 8.41 lb·in curing time 50% (TC 50) = 3:36 min curing time 90% (TC 90) = 13:01min scorch time (TS 1) = 1:03 min scorch time (TS 2) = 1:14 min |
||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
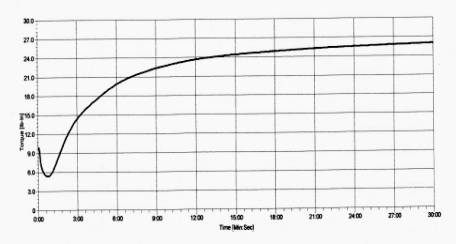
Figure 2b - Same rubber compound of Fig. 2a, containing 5% wt. (~ 20 phr) of XS_04 additive.
torque max = 26.01 lb·in torque min = 5.35 lb·in curing time 50% (TC 50) = 3:32 min curing time 90% (TC 90) = 13:00 min scorch time (TS 1) = 1:10 min scorch time (TS 2) = 1:22 min
|
||||||||||||||||||||||||||||
|
|
Figure 2 - Thermal vulcanization curves at 170°C (with numerical values of mim/max torque, scorch and curing times) of a sulfur-vulcanizing white EPDM rubber compound, as such and containing 5% wt. (~ 20 phr) of the microwave heating susceptor XS_04, respectively. Rubber compound preparations performed in a laboratory Banbury mixer (1-liter chamber); vulcanization tests performed by a R 100S Rheometer. |
|
|||||||||||||||||||||||||||
|
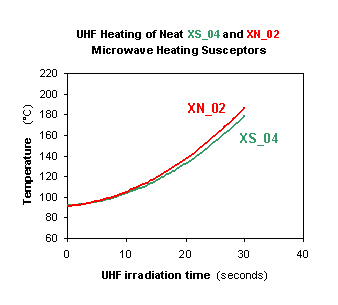
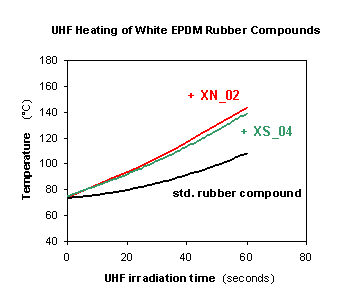
The UHF heatability of the novel microwave heating susceptors has been extensively monitored by a variety of laboratory experiments, as well as through extrusion & continuous UHF vulcanization pilot trials at external industrial rubber facilities. The efficacy of the novel additives is brightly evidenced by the simplest laboratory UHF heating tests carried out in conventional domestic microwave ovens, i.e. parallelepipedal multimode microwave resonant cavities similar to those commonly installed as multiple elements of the UHF tunnels in use at most rubber manufacturing facilities. The microwave heating effects detectable by such laboratory experiments are expected to be poorer (lower heating rates) with respect to those practically attainable from nominally equivalent industrial UHF ovens, equipped with cavity tuning devices (automatically or manually maximizing the power delivered to the materials being irradiated) absent in domestic UHF ovens. Figures 3 and 4 display the results of such laboratory microwave heating tests performed in standard domestic microwave ovens according to said "conservative" criteria. Figure 3 shows the UHF heatability of the two neat microwave heating susceptors XS_04 and XN_02; Figure 4 exemplifies the UHF heatability of two white EPDM rubber compounds containing 5% by weight of each of the two XS_04 and XN_02 additives, as compared to that of the same starting rubber compound free from them.
Table 2 - Costs & Benefits of the Novel Microwave Heating Susceptors as Additives in White & Colored Rubber Compounds, for Extrusion & Continuous UHF Vulcanization Processing of Rubber Seals and Hoses.
Comparative cost increases, productivity gains and energy + maintenance cost savings, with respect to conventional white & colored rubber compounds. Average estimates for additions of 4-5 % by weight (~20 phr) of XN additives to conventional white rubber compounds. Data resulting from factory tests performed on industrial extrusion & UHF vulcanization lines.
(*) depending on the characteristics and efficiency (operating conditions) of the UHF tunnel employed: number and output power of the UHF generators; volumetric microwave power density of the irradiation tunnel (mW/cu.cm) [multimode microwave resonant cavity or multiple cavities, or waveguide]; electromagnetic matching of the UHF generators with the rubber load being processed.
6. References
|
|||||||||||||||||||||||||||||